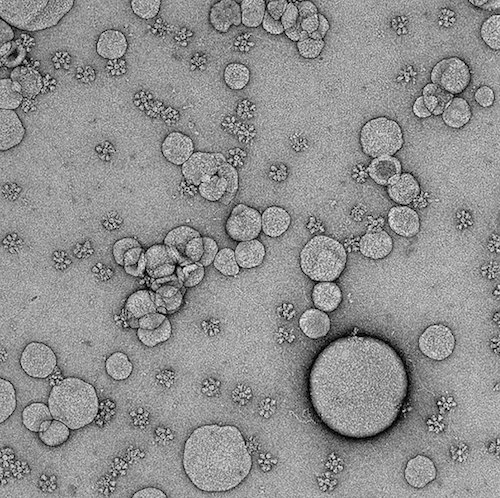
SpFN vaccine
A unique vaccine to protect against COVID-19 began Phase 1 clinical testing on April 6th at the Walter Reed Army Institute of Research (WRAIR) Clinical Trials Center. Scientists developed a nanoparticle vaccine based on a ferritin platform that offers a flexible approach to target multiple variants of SARS-CoV-2 and potentially other coronaviruses as well.
The vaccine, called spike ferritin nanoparticle (SpFN), stands out in the COVID-19 vaccine landscape. Its multi-faced sphere design allows repetitive, ordered presentation of the coronavirus spike protein to the immune system, a strategy that may help provide broader protection.
“Even before recent COVID-19 variants were identified, our team was concerned about the emergence of new coronaviruses in human populations, a threat that has been accelerating in recent years” said Dr. Kayvon Modjarrad, Director of EIDB, who leads the Army’s COVID vaccine research efforts. “That’s why we need a vaccine like this; one that has potential to protect broadly and proactively against multiple coronavirus species and strains.”
Pre-clinical studies(1) indicate that SpFN induces highly potent and broad neutralizing antibody responses against not only the initial strain of the virus that causes COVID-19 infection, but also against two major SARS-CoV-2 variants of concern and the rare, but more deadly, SARS-CoV-1 virus. The Phase 1 study is being conducted at WRAIR’s Clinical Trials Center in Silver Spring, Md., and will enroll 72 healthy adult volunteers ages 18-55.
“WRAIR prioritized community engagement early in the SpFN research and development process,” said Dr. Paul Scott, deputy director of EIDB. “As a result, a diverse group of volunteers will be involved in this Phase 1 trial and are committed to the overall goals of our vaccine strategy.”
Early in the pandemic, WRAIR structural biologist Dr. Gordon Joyce and his laboratory team generated an atomic-level view of the structure of the SARS-CoV-2 spike protein receptor binding domain—the part of the virus that attaches to the lung cells. “Seeing the viral spike protein in such detail provided a blueprint for vaccine design which allowed us to construct and test multiple vaccine candidates and identify one that generates a broad and protective immune response. You can’t predict the future, but you can be ready, and this vaccine is one of the first steps towards long-term protection.”
WRAIR’s vaccine is paired with an Army-patented adjuvant, ALFQ, to further boost the immune response. This adjuvant, developed by WRAIR scientists, has generated strong immune responses in preclinical studies and is being tested in a Phase 1 clinical trial with two malaria vaccine formulations.
“This first in human clinical trial of a novel vaccine for SARS-CoV-2 demonstrates the strength of WRAIR’s ability to very quickly transition exciting basic science discoveries to the clinic with the promise of developing a public health tool for long-term pandemic control,” said Dr. Nelson Michael, Director of WRAIR’s Center for Infectious Diseases Research. WRAIR is also providing expertise and support to the interagency U.S. federal government response aimed at accelerating the development of other COVID-19 vaccines, therapeutics and diagnostics.
“We are in this for the long haul,” said Modjarrad. “We have designed and positioned this platform as the next generation vaccine, one that paves the way for a universal vaccine to protect against not only the current virus, but also counter future variants, stopping them in their tracks before they can cause another pandemic.”
The trial is registered on clinicaltrials.gov: https://clinicaltrials.gov/ct2/show/NCT04784767
(1) Preclinical data on SpFN: https://www.biorxiv.org/content/10.1101/2021.03.24.436523v1
About the Study:
The Clinical Trial of SpFN is sponsored by the U.S. Army Medical Research and Development Command (USAMRDC). The vaccine was developed by the Walter Reed Army Institute of Research (WRAIR) Emerging Infectious Diseases Program with support from the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc (HJF). Funding was provided by the Defense Health Agency and was executed, in part, through a cooperative agreement between WRAIR and HJF (CA# W81XWH-18-2-0040).

